Immune Cell-based Therapies for COVID-19: Current Clinical Trial Landscape Data
One element of COVID-19 is a hyper-response of the patient’s immune system, which in turn leads to lung injury and acute respiratory distress syndrome.1 At the same time, appropriate immune response is decreased due to immune evasion by the virus. 1,2 Treatment with immune cell-based adoptive therapies is being investigated to combat inflammation to fight SARS-CoV-2 (i.e., T regulatory and NK cells).2 Here, we review the current clinical trial landscape utilizing immune cell-based therapies.
To obtain the results, we queried the clinicaltrials.gov database using the following parameters and search terms:
As of August 4, 2020, the clinicaltrials.gov website had 15 total relevant trials. 6 Trials are registered as Phase 1, 7 trials are at Phase 1/2, and 1 trial is at Phase 2 trial (1 trial has no phase designation listed).
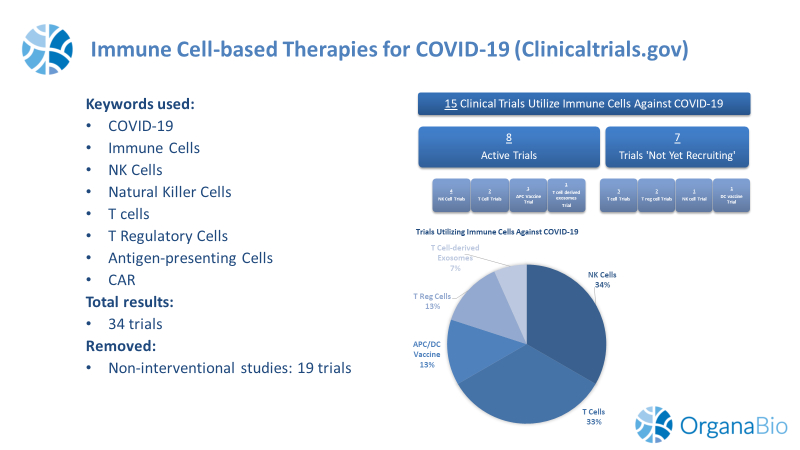
Currently, there are 8 “active” (i.e., Recruiting; Active, not recruiting; Not yet recruiting) immune cell-based trials for COVID-19. 50% of these trials are evaluating allogeneic NK cells and 25% are treating patients with T cells. Trial location is widespread across the globe, with the largest number of active trials located in North America (53%) followed by East Asia (33%).
One of the Phase 1/2 trials “Natural Killer Cell (CYNK-001) Infusions in Adults With COVID-19 (CYNK-001-COVID-19)” (NCT04365101; Celularity Inc., IDRI, Lung Biotechnology PBC) is investigating the therapeutic use of CYNK-001, an allogeneic, off-the-shelf NK cell product, developed from placental hematopoietic stem cells.3 CYNK-001 received FDA clearance back in April, allowing the Phase 1/2 to enroll up to 86 COVID-19 patients.3 Another upcoming trial to watch is of Caladrius’ CLBS119 for the repair of lung damage found in patients with severe COVID-19 infection who experienced respiratory failure.4 CLBS119, a CD34+ cell therapy product, is planned to be in a clinical trial for COVID-19 in the coming months.4 Check in next month for an updated version of this post!
Immunotherapeutic strategies against COVID-19 in clearing the infection while preventing the harm of immunoinflammatory responses are challenging. The right immune cell therapy will depend on the individual patient and disease stage, but insight and understanding from clinical trial data will help guide towards effective therapy and treatment course.
References
- Remy KE, Brakenridge SC, Francois B, et al. Immunotherapies for COVID-19: lessons learned from sepsis [published online ahead of print, 2020 Apr 28]. Lancet Respir Med. 2020;S2213-2600(20)30217-4.
- Guihot A, Litvinova E, Autran B, Debré P, Vieillard V. Cell-Mediated Immune Responses to COVID-19 Infection. Front Immunol. 2020;11:1662. Published 2020 Jul 3.
- https://www.genengnews.com/covid-19-candidates/celularity-and-sorrento-therapeutics-cynk-001/
- https://bionj.org/event/bionjs-covid-19-rapid-fire-research-showcase






