Some say that CAR-T (Chimeric Antigen Receptor T cell) revolutionized cell therapy and transformed the manufacturing technologies of the industry. Following the approval and success of the CD19 CAR-T, many more tumor-specific molecules are being explored and targeted by CAR technology. The most exciting developments are allogeneic CAR-T which may be easier to manufacture in large quantities and in a more economic and efficient way. Our ImmunoPAC™-T-CB T cells are perfect starting material for these innovative “off-the-shelf” therapeutics. Here we will explore the basic biology behind the story of T lymphocytes.
Once T cells complete their development in the thymus, they enter the bloodstream, travel to lymphoid organs, and continue to recirculate. Mature T cells receive survival signals during recirculation via the T cell receptor (TCR) recognizing self-peptide/self-MHC complexes and from cytokines. These are termed naïve T cells. In order to become effector T cells, naïve T cells must be activated by a foreign peptide/self-MHC complex (along with other co-stimulatory molecules, for example CD28) displayed on an antigen-presenting cell (such as dendritic cells and macrophages). Depending on the peptide origin, T cells differentiate into CD8+ cytotoxic T cells (CTLs) or CD4+ helper T cells and begin to proliferate. While CD8 T cells directly kill, CD4 T cells assist in activation of other immune cells. During this primary immune response, long lived memory T cells are also generated to provide protection from subsequent challenges by the same foreign antigen.
Within the CD4 T cell population, there are regulatory T cells as well. These cells are characterized by the expression of CD25, secretion of transforming growth factor beta (TGF-β) and interleukin 10 (IL-10). Regulatory T cells respond to specific stimuli and their pattern of cytokine secretion inhibits CD4 T helper cell development and response, resulting in low levels of antibody production by B cells, and significantly decreases inflammatory T cell responses. Clinical translation of regulatory T cell therapy and the concept of regulatory T cell-based immunotherapy have been shown to facilitate tolerance in autoimmune settings and transplantation1.
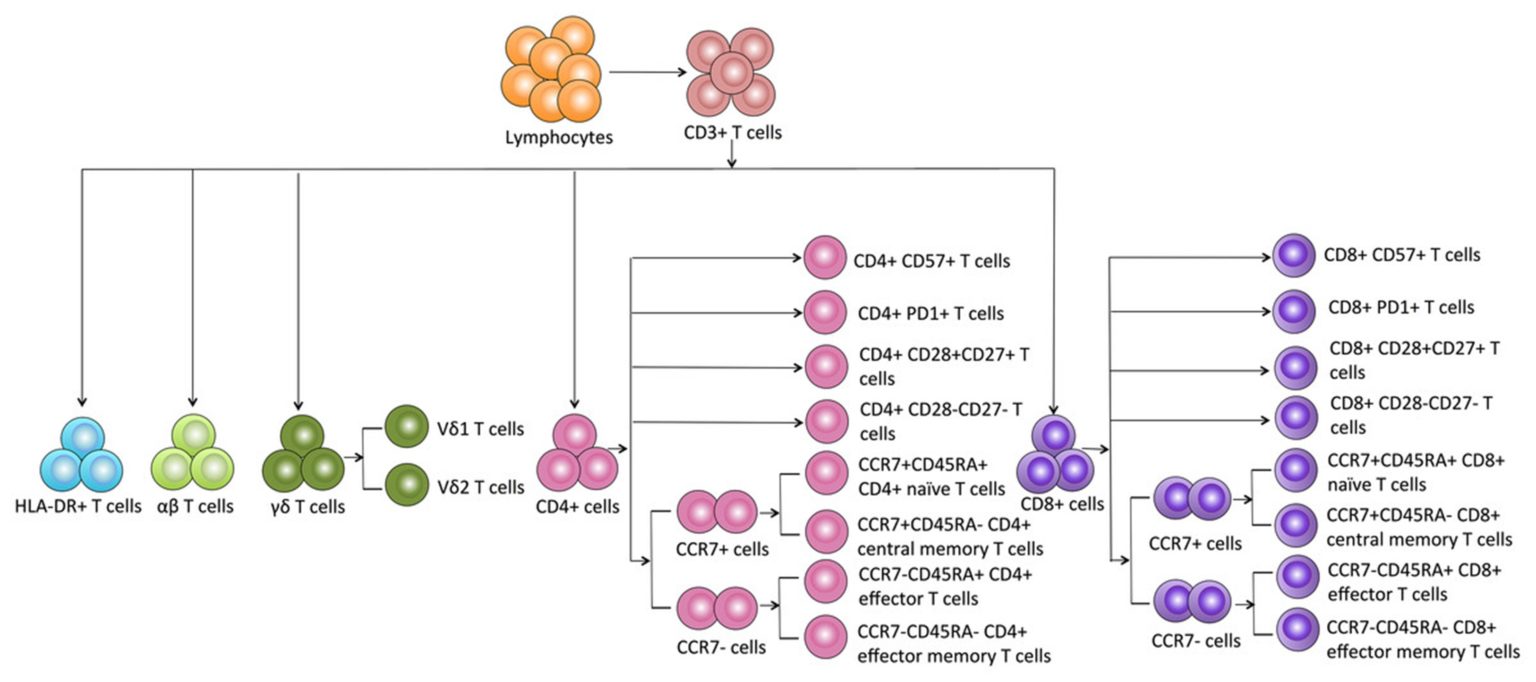
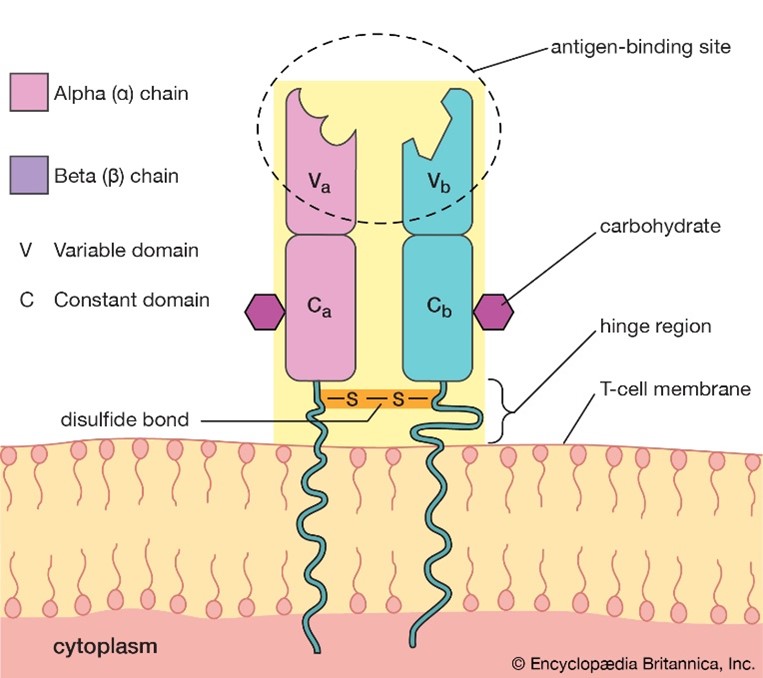
As mentioned already, the TCR on T cells only recognizes foreign antigens that are displayed in the context of self-MHC. As might be expected from this function, TCRs are highly variable. Each TCR consists of two polypeptides chains, TCRɑ and TCRβ, linked together on the surface of a T cell, and each chain is composed of constant (C) and variable (V) regions. A minor population of T cells expresses TCR made up of γ and δ polypeptide chains instead of TCRɑβ. Although it is thought that the ɑβ and γδ T cells arise from a common precursor, they are very different. γδ T cells can recognize free antigens that are not in the context of MHC and are considered “innate-like” T cells. These properties make γδ T cells attractive for universal cell therapy by overcoming the challenges of allogeneic immunotherapies2. Another unique lymphocyte population is the NKT cell, which expresses both TCR and NK cell receptors. More on this will be described in our next blog.
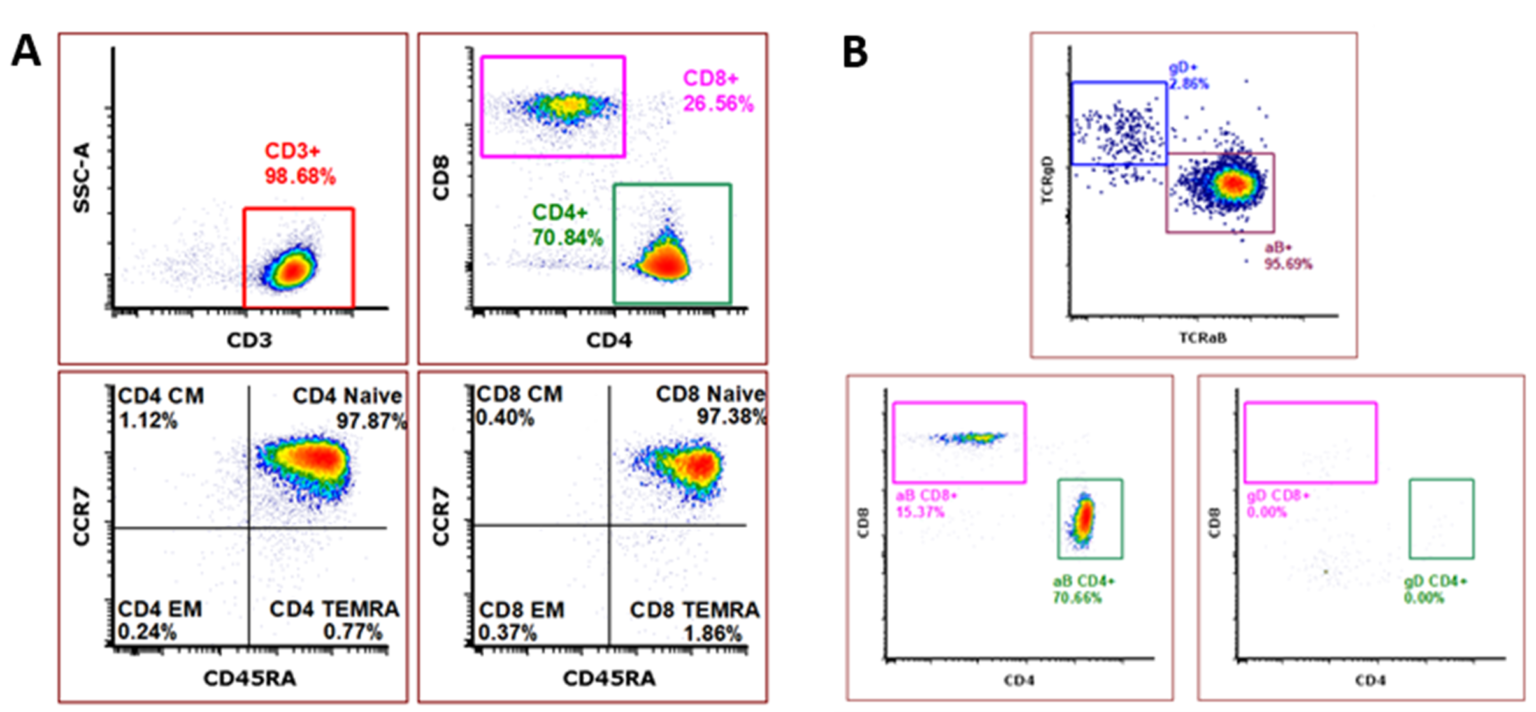
Our ImmunoPAC™-T-CB is a pure, naïve, and highly viable cryopreserved primary T cell product derived from human umbilical cord blood. In comparison to adult peripheral blood-derived T cells, cord blood-derived T cells have a higher CD4:CD8 ratio and a higher percentage of naïve cells3. This unique immature and naïve cell population has been shown to quickly propagate into memory cells upon stimulation3,4. Additionally, cord blood T cells have been shown to facilitate enhanced antitumor responses in comparison to peripheral blood-derived cells4,5. The inherent nature of cord blood cells to have reduced risk of causing graft-versus-host disease (GvHD), along with these defining characteristics, make cord blood T cells an appealing option for the development of gene-modified and non-modified allogeneic cell therapies4. Our ImmunoPAC™-T-CB products consistently demonstrate a pure CD3 population with a phenotype demonstrating high CD4:CD8 ratio, naïve phenotype of both CD4 and CD8 subpopulations, and a distinct TCRγ/δ population lacking CD4 and CD8 expression.
References
- Fraser H, Safinia N, Grageda N, Thirkell S, Lowe K, Fry LJ, Scottá C, Hope A, Fisher C, Hilton R, Game D, Harden P, Bushell A, Wood K, Lechler RI, Lombardi G. A Rapamycin-Based GMP-Compatible Process for the Isolation and Expansion of Regulatory T Cells for Clinical Trials. Mol Ther Methods Clin Dev. 2018 Jan 31;8:198-209. doi: 10.1016/j.omtm.2018.01.006. PMID: 29552576; PMCID: PMC5850906.
- Yazdanifar M, Barbarito G, Bertaina A, Airoldi I. γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells. 2020;9(5):1305. Published 2020 May 24. doi:10.3390/cells9051305
- Yun, Hyun Don, et al. “Clinical Relevance of Immunobiology in Umbilical Cord Blood Transplantation.” Journal of Clinical Medicine, vol. 8, no. 11, 2019, p. 1968., doi:10.3390/jcm8111968.
- Presti, Vania Lo, et al. “Use of Cord Blood Derived T-Cells in Cancer Immunotherapy: Milestones Achieved and Future Perspectives.” Expert Review of Hematology, vol. 11, no. 3, 2018, pp. 209–218., doi:10.1080/17474086.2018.1431119.
- Hiwarkar, Prashant, et al. “Cord Blood T Cells Mediate Enhanced Antitumor Effects Compared with Adult Peripheral Blood T Cells.” Blood, vol. 126, no. 26, 2015, pp. 2882–2891., doi:10.1182/blood-2015-06-654780.






