With the rapid advancement of cell therapies, there is a demand for cellular starting materials that are robust, reproducible, well-characterized, and clinically relevant. Many adoptive cell therapies are based on lymphocytes and hematopoietic stem cells. Whether it’s allogeneic CAR-T or hematopoietic stem cell transplantation (HSCT), clinical development and commercial scale up and scale out require a high quantity of high quality cells as starting materials.
Cord blood (CB) HSCT has been around for over 30 years. It has been shown to have practical, biological, and clinical benefits compared to bone marrow. In contrast to other allogeneic cell sources, CB is readily available as frozen, “off the shelf” product. Additionally, search time for CB is reduced compared to unrelated bone marrow or peripheral blood donors (search time for unrelated CB is ~12 days and bone marrow ~3–4 months).1 CB also requires less stringent HLA matching and results in lower incidence of graft-versus-host disease (GVHD). These characteristics have made CB more accessible. Over the years, the use of CB-derived cells has resulted in a significant positive impact on treatment outcomes.
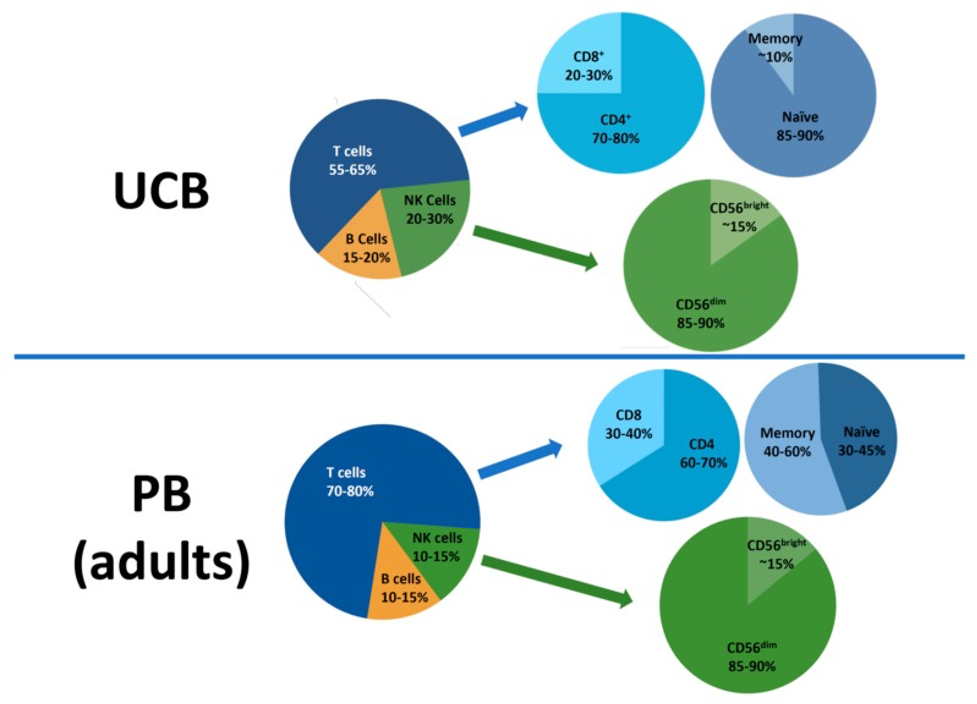
As opposed to bone marrow and peripheral blood, most lymphocytes in CB are immature and naïve, antigen-inexperienced cells. T cells in CB have a higher capacity to be transformed into memory T cells and have been shown to be an excellent source for genetically modified T cells.2,3,6 CB-derived T cells also exhibit enhanced anti-tumor activity with a high tumor-infiltrating CD8/Regulatory T cells (Tregs) ratio, compared to adult peripheral blood T cells.4 CB-derived virus-specific T cells were shown to be safely and effectively administered to HSCT recipients in order to prevent and treat viral reactivation.5 The unique γδ T cell subset can also be found in cord blood. These “innate-like” T cells have anti-viral and anti-tumor activities in major histocompatibility complex (MHC)-independent context.7 CB is also a rich source of a unique, immature Natural Killer (NK) cell population that can mature into potent NK cells with high proliferation and cytotoxicity capacities which lead to an impressive graft-versus-leukemia activity.8 CB-derived NK cells can be expanded >1000-fold and reliably produced for clinical use, such as for manufacturing of CAR-NK.9
Despite the numerous benefits to utilizing CB-derived lymphocytes as starting material for cell therapies, there are some challenges. One of the biggest challenges that researchers encounter with using CB-derived cells is cell number.10 In order to develop, manufacture, and dose patients with cellular-based treatments, the average cell number from a single cord blood unit is not sufficient. It is often necessary to use two units of cord blood, which becomes very expensive (estimated ~$80,000). CB units are also kept frozen for years before use, and this results in a lower viable cell yield upon thawing. Therefore, many researchers are required to spend time and resources on ex-vivo expansion of cells derived from CB, which is not always cost-efficient, and can also prolong time to therapy deployment to patients.
Due to the challenges around use of CB-derived lymphocytes, some scientists prefer to use adult peripheral blood (i.e., circulating whole blood) as a starting material. There are clinical benefits to adult-derived lymphocytes compared to CB-derived lymphocytes. As opposed to the delay in CB engraftment and immune reconstitution, recipients of peripheral blood transplantation experience normal recovery rates. Studies have shown that hematopoietic recovery after peripheral blood stem cell transplantation is also faster compared to CB HSCT.11 The ability to reconstitute the immune system in a timely manner has implications which include decreased risk of infections in the recipient, lower morbidity and mortality, as well as improved treatment outcome and efficacy.12
No matter what your preferred source of raw material is, to successfully develop cell and gene therapies, it is important to have a steady and reliable source of high quality starting materials. At OrganaBio, we address this mission-critical industry need by isolating, characterizing and providing a variety of exceptional cell products to cell therapy developers. We also understand the benefits of having ready access to a variety of tissues and cell sources on basic science, R&D, cell therapy preclinical development and testing, and manufacturing. Hence, we are consistently broadening our portfolio of products to best serve the industry and meet unmet needs.
You can learn more about our products here. You can also sign up to receive alerts on new product launches and industry news by interacting with us in the chat box below.
References:
- Confer D, Robinett P. The US National Marrow Donor Program role in unrelated donor hematopoietic cell transplantation. Bone Marrow Transplant. 2008 Aug;42 Suppl 1:S3-S5. doi: 10.1038/bmt.2008.102.
- Ma Q, Garber HR, Lu S, et al. A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells. Cytotherapy. 2016;18(8):985–994. doi:10.1016/j.jcyt.2016.05.001
- Lo Presti V, Nierkens S, Boelens JJ, van Til NP. Use of cord blood derived T-cells in cancer immunotherapy: milestones achieved and future perspectives. Expert Rev Hematol. 2018;11(3):209–218. doi:10.1080/17474086.2018.1431119
- Hiwarkar P, Qasim W, Ricciardelli I, et al. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood. 2015;126(26):2882–2891. doi:10.1182/blood-2015-06-654780
- Abraham AA, John TD, Keller MD, et al. Safety and feasibility of virus-specific T cells derived from umbilical cord blood in cord blood transplant recipients [published correction appears in Blood Adv. 2019 Aug 27;3(16):2453]. Blood Adv. 2019;3(14):2057–2068. doi:10.1182/bloodadvances.2019000201
- Yun HD, Varma A, Hussain MJ, Nathan S, Brunstein C. Clinical Relevance of Immunobiology in Umbilical Cord Blood Transplantation. J Clin Med. 2019;8(11):1968. Published 2019 Nov 14. doi:10.3390/jcm8111968
- Berglund S, Gaballa A, et al. “Expansion of Gammadelta T Cells from Cord Blood: A Therapeutical Possibility.” Stem Cells Int. 2018 Mar 7;2018:8529104. doi: 10.1155/2018/8529104.
- Mehta RS, Rezvani K, Olson A, et al. Novel Techniques for Ex Vivo Expansion of Cord Blood: Clinical Trials. Front Med (Lausanne). 2015;2:89. Published 2015 Dec 11. doi:10.3389/fmed.2015.00089
- Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545–553. doi:10.1056/NEJMoa1910607
- Barker JN, Kempenich J, Kurtzberg J, et al. CD34+ cell content of 126 341 cord blood units in the US inventory: implications for transplantation and banking. Blood Adv. 2019;3(8):1267-1271. doi:10.1182/bloodadvances.2018029157
- Brunstein CG, Gutman JA, Weisdorf DJ et al. Allogeneic hematopoetic cell transplantation for hematologic malignancy: Relative risks and benefits of double umbilical cord blood. Blood 2010;116:4693–4699
- Takagi, S., Ogura, S., Araoka, H. et al. The impact of graft cell source on bloodstream infection in the first 100 days after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant (2021).