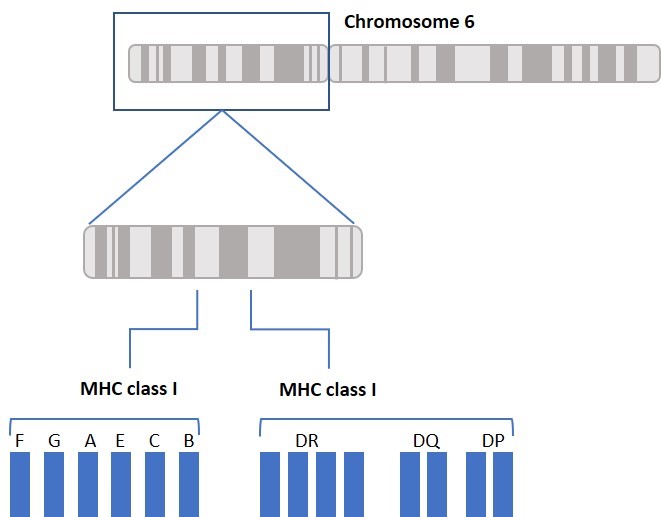
What do a woman suffering from Psoriasis, a man dying after acute rejection of kidney transplant, and a child suffering from Pulmonary Tuberculosis due to susceptibility to mycobacteria infection all have in common? These situations and conditions all involve the Human Leukocyte Antigen (HLA) System. Our body’s immune system uses HLA to distinguish between our own and foreign cells, and between healthy and infected cells. Think of HLA as a person’s signature. HLA proteins are encoded by a cluster of Major Histocompatibility Complex (MHC) genes located on chromosome 6 and are highly variable. While HLA is often associated with bone marrow and organ transplant, HLA typing is also used to identify disease-related biomarkers and has a role in vaccine development.
The main function of HLA is to educate our immune cells to distinguish between self and non-self. HLA molecules also play a role in initiating and coordinating immune response by binding and presenting antigens. HLA genes that involve the immune system belong to two major groups: MHC class I and MHC class II. Class I molecules are encoded by HLA-A, HLA-B, and HLA-C genes/loci and present intracellular antigens originating from viruses or tumors to cytotoxic CD8+ T cells. Class I HLA-G proteins play a role in immune tolerance (like maintaining fetal-maternal tolerance1), and Class I HLA-E proteins react with natural killer (NK) cells. Activation or inhibition of NK cells is achieved through crosstalk between killer immunoglobulin-like receptors (KIRs) on NK cells and HLA. (More about KIRs to come in a future blog.) Class II molecules are encoded by HLA-DP, HLA-DQ, HLA-DR, HLA-DM, and HLA-DO, and are mainly found on antigen presenting cells and present extracellular antigens to CD4+ T helper cells. MHC class III genes/loci do not encode HLA molecules. The MHC class III region encodes complement components, tumor necrosis factors (TNFs), and others (Figure 1).
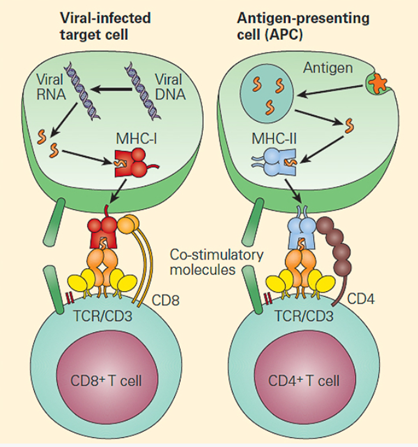
([Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012)
Everyone’s HLA complex consists of unique variants of class I and class II genes, also called alleles. The string of alleles is referred to as a haplotype. We inherit one haplotype from each parent, and the two form each person’s HLA genotype. HLA typing could be done based on serology, which detects anti-HLA antibodies, or by DNA sequencing, which provides specific allele information. We use high resolution DNA sequencing to provide HLA genotypes on all of our cell products. In allogeneic organ or cell transplantation, it is much easier to find an HLA matched donor in the patient’s family. For siblings, the probability of having 2 identical haplotypes is 25%, the probability of having one identical haplotype is 50%, and the probability of having two different haplotypes is 25%. The probability of finding an unrelated matched donor depends on ethnicity and ranges between 16% (for black people of South or Central American descent) and 75% (for white people of European descent). 4
When it comes to organ or bone marrow transplantation, an optimal unrelated donor will require at least 8/8 allele match with the recipient at the HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci. Various studies revealed that these specific genes are associated with graft rejection and patient survival compared to the rest of the HLA genes.2,3 A 7/8 allele match (i.e., one mismatch) reduces the 5-year overall survival rate by about 8%. Matching requirements for cord blood are less stringent (6/6 matching of HLA-A and HLA-B and HLA-DRB1). This is due to the increased proliferative capability of cord blood hematopoietic stem cells and the immature nature of cord blood immune cells, which lowers the risk for rejection and graft-versus-host (GvHD) disease.5-7 GvHD occurs when the donor’s cells attack the recipient’s healthy cells. This response is due to cross-reactivity in an HLA mismatched transplantation.
Just as in the case of organ and tissue transplantation, it is important to consider HLA matching when developing allogeneic cell therapies, such as mesenchymal stem cell (MSC) and immune cell therapies. Although MSCs express low levels of HLA I and low/no HLA II and are sometimes considered immune-privileged and better tolerated, immune reactions should still be considered. It has been reported that bone marrow-derived allogeneic mismatched MSCs elicited an immune reaction in vivo. Therefore, in order to develop safe and effective treatments, we must consider the immunological interactions between donor and recipient cells.8,9 All of OrganaBio’s cell products are HLA typed in order to better inform researchers and to ensure safe and effective cell therapies.
Some specific HLA types are associated with protective immunity versus susceptibility to infectious diseases. HLA genes have evolved through natural selection by selective pressure from infectious pathogens, with malaria being one of them. HIV vaccine development relies on specific HLA types targeted by vaccine peptides which induce protective immunity. HLA typing has also led to some improvement in the diagnosis of celiac disease and type 1 diabetes. Additionally, several autoimmune diseases have been correlated with specific HLA-B27 alleles. More on this in a future post.
Allele – A variant form of a gene, or locus
Haplotype– The combination of alleles from two or more loci located on the same chromosome
Genotype – The combination of two haplotypes; one from each parent, inherited by an individual
References
- Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL. HLA-G: at the interface of maternal-fetal tolerance. Trends Immunol. (2017) 38:272–86.
- Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation.
- Horan J, Wang T, Haagenson M, et al. Evaluation of HLA matching in unrelated hematopoietic stem cell transplantation for nonmalignant disorders. Blood. 2012; 120:2918–24.
- Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339-348.
- Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009; 113:1631–8.
- Gluckman E, Rocha V, Arcese W, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004; 32:397–407.
- Dessels C, Alessandrini M, Pepper MS. Factors Influencing the Umbilical Cord Blood Stem Cell Industry: An Evolving Treatment Landscape. Stem Cells Transl Med. 2018;7(9):643-650. doi:10.1002/sctm.17-0244
- Isakova I.A., Lanclos C., Bruhn J., Kuroda M.J., Baker K.C., Krishnappa V., Phinney D.G. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS ONE. 2014;9:e87238.
- Pezzanite L.M., Fortier L.A., Antczak D.F., Cassano J.M., Brosnahan M.M., Miller D., Schnabel L.V. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res. Ther. 2015;6:1–11.






