At the beginning of August, we searched the clinicaltrials.gov database to learn more about the U.S.-based clinical trial landscape of mesenchymal stromal cell (MSC) based therapies for COVID-19. The original search, conducted on July 31, 2020, revealed an active landscape filled with 53 “active” clinical trials (i.e., Available; Recruiting; Active, not recruiting; Enrolling by invitation).
For this blog post, we revisited our search parameters to provide you with an update on previous research. To obtain the results, we queried the clinicaltrials.gov database using the following parameters and search terms:
| Intervention/treatment: | “Mesenchymal stem cell” OR “Mesenchymal Stromal Cell” OR “MSC” |
| Condition or Disease: | COVID |
As of October 27, 2020, the clinicaltrials.gov website had 59 “active” MSC-based trials for COVID-19 (email us for the spreadsheet). Within the last 3 months, two trials have since been completed and 8 new trials have been added. See Table 1 for a summary of these changes.
Table 1 | Recently Completed and New MSC-based Clinical Trials for COVID-19
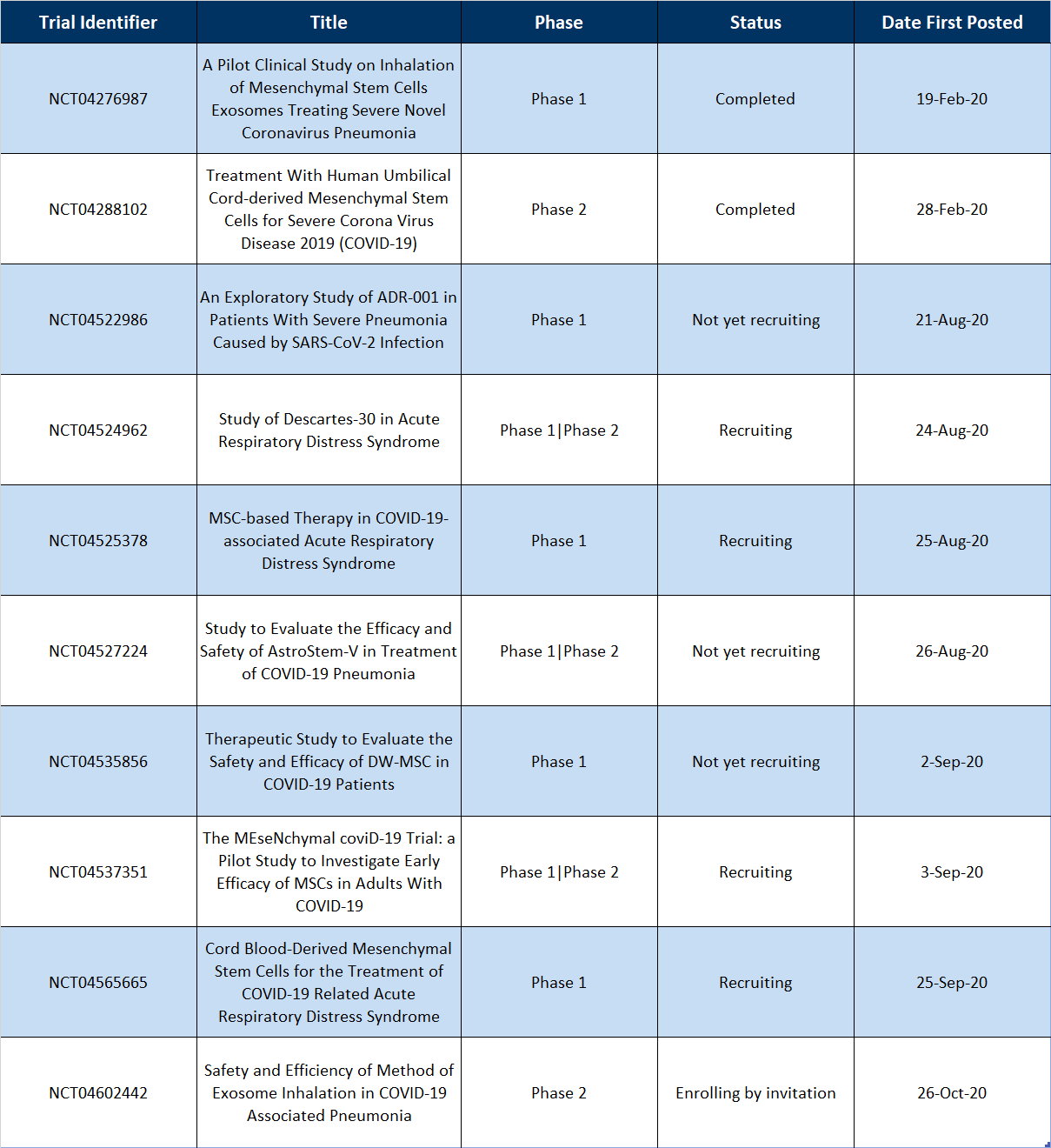
The two completed trials, “A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia” (NCT04276987) and “Treatment With Human Umbilical Cord-derived Mesenchymal Stem Cells for Severe Corona Virus Disease 2019 (COVID-19)” (NCT04288102) had Last Updated Posts (“The most recent date on which changes to a study record were made available on ClinicalTrials.gov. There may be a delay between when the changes were submitted to ClinicalTrials.gov by the study’s sponsor or investigator (the last update submitted date) and the last update posted date.”) of September 7, 2020 and August 19, 2020, respectively. If this is assumed to be the date of which the study was updated to “completed”, then the trials took roughly 8 and 7 months to complete, respectively. This is especially impressive since the first trial enrolled 24 patients and the second enrolled 100.
For the newly posted studies, 62.5% (5 of 8) appear to take allogeneic-based approaches for their therapeutic (NCT04276987, NCT04522986, and NCT04602442 do not make a mention ‘allogeneic’ or ‘autologous’). Allogeneic approaches allow for “off-the-shelf therapies” that can quickly be delivered to patients in need. Given the grave state some can fall into during this disease, an allogeneic approach makes the most sense for delivering a therapeutic quickly. Allogeneic approaches also avoid the additional risk of an invasive procedure to harvest cells (in an already sick patient for that matter) and the additional time needed to expand the cells.
Also interesting is the fact that the MSCs used in these studies are derived from a variety of sources. “An Exploratory Study of ADR-001 in Patients With Severe Pneumonia Caused by SARS-CoV-2 Infection” (NCT04276987) and “Study to Evaluate the Efficacy and Safety of AstroStem-V in Treatment of COVID-19 Pneumonia” (NCT04527224) are using adipose-derived MSCs, “Therapeutic Study to Evaluate the Safety and Efficacy of DW-MSC in COVID-19 Patients” (NCT04535856) “The MEseNchymal coviD-19 Trial: a Pilot Study to Investigate Early Efficacy of MSCs in Adults With COVID-19” (NCT04537351) are using iPSC-derived MSCs, and “Cord Blood-Derived Mesenchymal Stem Cells for the Treatment of COVID-19 Related Acute Respiratory Distress Syndrome” (NCT04565665) are using umbilical cord blood-derived MSCs. Only one trial, “Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia” (NCT04602442), is using MSC-derived exosomes instead of MSCs themselves for the therapy.
What are your thoughts on using MSCs for COVID-19? Feel free to leave a comment below and check back next quarter for another update.






