Advanced cell-based therapies, such as chimeric antigen receptor (CAR)-T cell therapies, are changing the landscape of healthcare, expanding treatment options for patients beyond previous standards of care in major disease areas. To ensure successful cell therapy outcomes, the timely and reliable delivery of high-quality cellular materials to drug developers with active clinical trials and, ultimately, patients are crucial. However, difficulties arise in managing the vein-to-vein workflows for these advanced therapies given the complexities of collecting samples, processing specific cell types, and then shipping cryopreserved materials within a narrow window of time. Furthermore, clinical trial participants and patient populations are geographically separated from collection and manufacturing facilities, where cells are isolated and engineered. Proximity, chain of custody, and sample integrity introduce many potential points of failure.
While short-term storage at temperatures of 2-8°C for 2–4 days is feasible for fresh apheresis material, extending the shelf life outside of this limited window leads to significant metabolic decline1,2. Therefore, a more practical, reliable, and long-term solution for storage and transport is cryopreservation. At temperatures below −130°C, metabolic changes are mitigated, effectively preserving the viability and functionality of cellular material. Added benefits of including an established cryogenic cycle to the cell therapy manufacturing workflow allow for an extension on the shelf life of cellular material post-collection, leading to higher-quality and more consistent cell therapy products. In fact, cryopreservation has already been integrated into the manufacturing workflow of many FDA-approved cell therapies, including Novartis’ CD19-directed CAR-T cell therapy Kymriah®, to simplify logistics and improve patient access3.
Challenges in Navigating Cold Chain Logistics
The typical cell therapy workflow involves the clinic or hospital where apheresis material is collected from the clinical trial participant or patient. The apheresis material is then processed, enriching the sample based on the trial’s requirements. The subsequent steps of cryopreservation following sample processing present new challenges as many of the clinical collection sites lack the necessary infrastructure—and often technical expertise—required for effective cryopreservation, not to mention the capacity to manage other aspects of the cold chain.
Consequently, clinical trial samples and critical starting material may need to be transported from the collection site to a centralized facility for cryopreservation even before reaching the manufacturing facility. In most cases, samples are shipped to contract research organizations (CROs) to facilitate this key step. The logistical complexities involved in these transit steps and outsourcing work to multiple CROs introduce significant risk – to the trial being conducted and the final therapeutic product. Unforeseen disruptions in the cold chain logistics and a lack of proven expertise could compromise cellular viability, thereby jeopardizing treatment efficacy and patient safety.
In summary, these issues place many clinical trial sponsors and drug developers in uncharted territory as they attempt to bridge the gap by managing their own cold chain logistics for starting materials, a task that is often complex, challenging, and requires costly infrastructure buildouts. Moreover, Sponsors’ materials must be maintained at the appropriate temperatures in transit between sites – a process that takes several days and involves multiple couriers, particularly in situations requiring air freight across international borders (Figure 1). This fragmented chain of custody is difficult to manage. Securing proper storage, handling samples, and recording each data point in the process are required at each transit step to ensure sample integrity. Therefore, an efficient cold chain management system is necessary to de-risk cell therapy logistics and safeguard materials through every step of the workflow to ensure that safe and effective treatments can be delivered to patients in need.
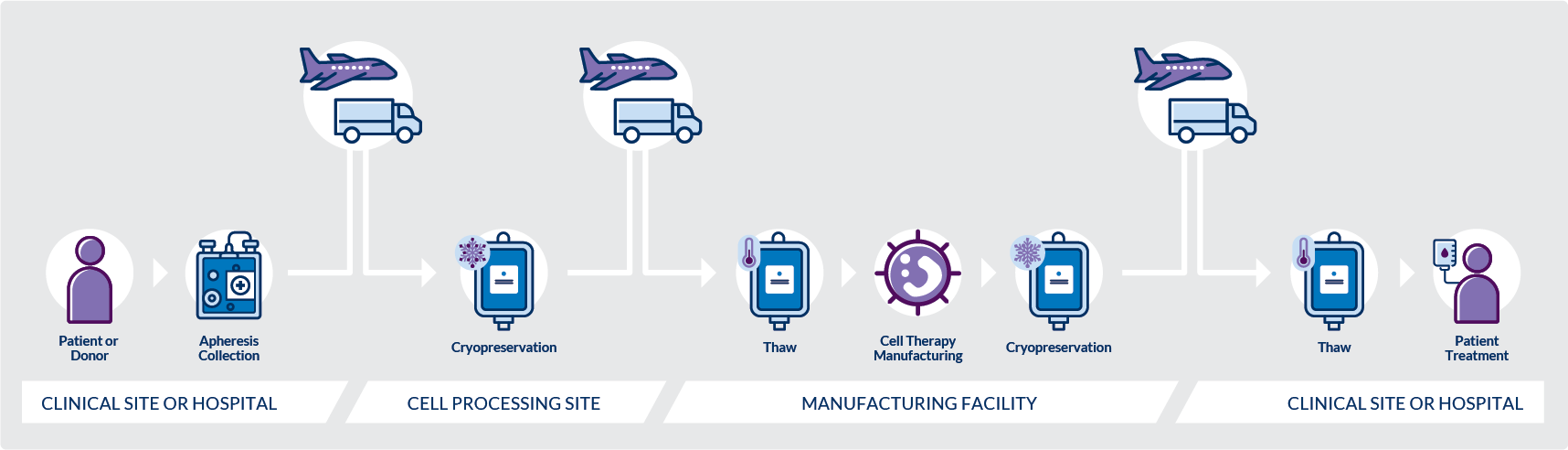 Figure 1. A typical workflow involves the movement of time-critical shipments between several sites with at least three transfer points, using multiple couriers, which requires seamless coordination and management to execute. This fragmented chain of custody introduces significant risk to the efficacy of the final product.
Figure 1. A typical workflow involves the movement of time-critical shipments between several sites with at least three transfer points, using multiple couriers, which requires seamless coordination and management to execute. This fragmented chain of custody introduces significant risk to the efficacy of the final product.
At OrganaBio, our vertically integrated solutions coupled to our hub processing model are specifically designed to provide cohesion across the primary transfer points for sample collection and cryopreservation, and end-to-end shipping logistics. By completing all steps by OrganaBio, inherent risks across the entirety of the process are mitigated, while the benefits are fully realized. Clients across the globe partner with OrganaBio to provide critical raw material for ongoing clinical trials and to play a key role in collecting samples from participants in active clinical trials. OrganaBio’s cold chain logistics and client-focused project management ensure near–perfect results and a seamless transition between all checkpoints – with optimal quality and transparency every step of the way.
Contact our team at [email protected] to learn more.
References
- Meneghel J, Kilbride P, Morris GJ. Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies-A Review. Front Med (Lausanne). 2020; 7:592242. Published 2020 Nov 26. doi:10.3389/fmed.2020.592242
- Qayed M, McGuirk JP, Myers GD, et al. Leukapheresis guidance and best practices for optimal chimeric antigen receptor T-cell manufacturing. Cytotherapy. 2022;24(9):869-878. doi:10.1016/j.jcyt.2022.05.003
- Tyagarajan S, Schmitt D, Acker C, Rutjens E. Autologous cryopreserved leukapheresis cellular material for chimeric antigen receptor-T cell manufacture. Cytotherapy. 2019;21(12):1198-1205. doi:10.1016/j.jcyt.2019.10.005






