MSC-Based Therapies for COVID-19: Current Clinical Trial Landscape Data
Mesenchymal stem/stromal cells (MSCs) are emerging as a potential treatment modality for COVID-191-3. MSCs have immunomodulatory properties that can be used to combat the inflammatory response caused by the SARS-CoV-2 coronavirus, and the United States Food and Drug Administration (FDA) has granted accelerated approvals to several MSC-based clinical trials for COVID-19. This brief blog post summarizes the current MSC COVID-19 clinical trial landscape based on data from clinicaltrials.gov.
To obtain the results, we queried the clinicaltrials.gov database using the following parameters and search terms:
| Intervention/treatment: | “Mesenchymal stem cell” OR “Mesenchymal Stromal Cell” OR “MSC” |
| Condition or Diseases: | COVID |
As of July 31, 2020, the clinicaltrials.gov website had 57 total trials (email us for the spreadsheet). The earliest recorded trial, “Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST) (COVID-19)”, was first published in February 3, 2017 (link). However, the first COVID-19 case was reported on December 31, 2019 in Wuhan, China4. The aforementioned article was updated on March 20, 2020 to include COVID-19 background data, so it is likely that the trial was initially published to address other acute respiratory distress syndromes (ARDS) and amended this year with the emergence of COVID-19. The next earliest trial, “Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With COVID-19”, was first posted on February 5, 2020 and is arguably the first recorded COVID-19 trial on clinicaltrials.gov.
Currently, there are 53 “active” (i.e., Available; Recruiting; Active, not recruiting; Enrolling by invitation) MSC-based trials for COVID-19 (See figure 1, below). 83% of these trials are in Phase 1 (21%), Phase 2 (28%), or Phase 1/2 (34%). 4% of trials are in later stages of clinical testing (Phase 2/3 or Phase 3). Trial location is widespread across the globe, with the largest number of active trials located in North America (38%), followed by Europe (23%) and East Asia (19%). This is a significant shift from several months ago when all active clinical trials for COVID-19 were in East Asia, and more specifically, China.
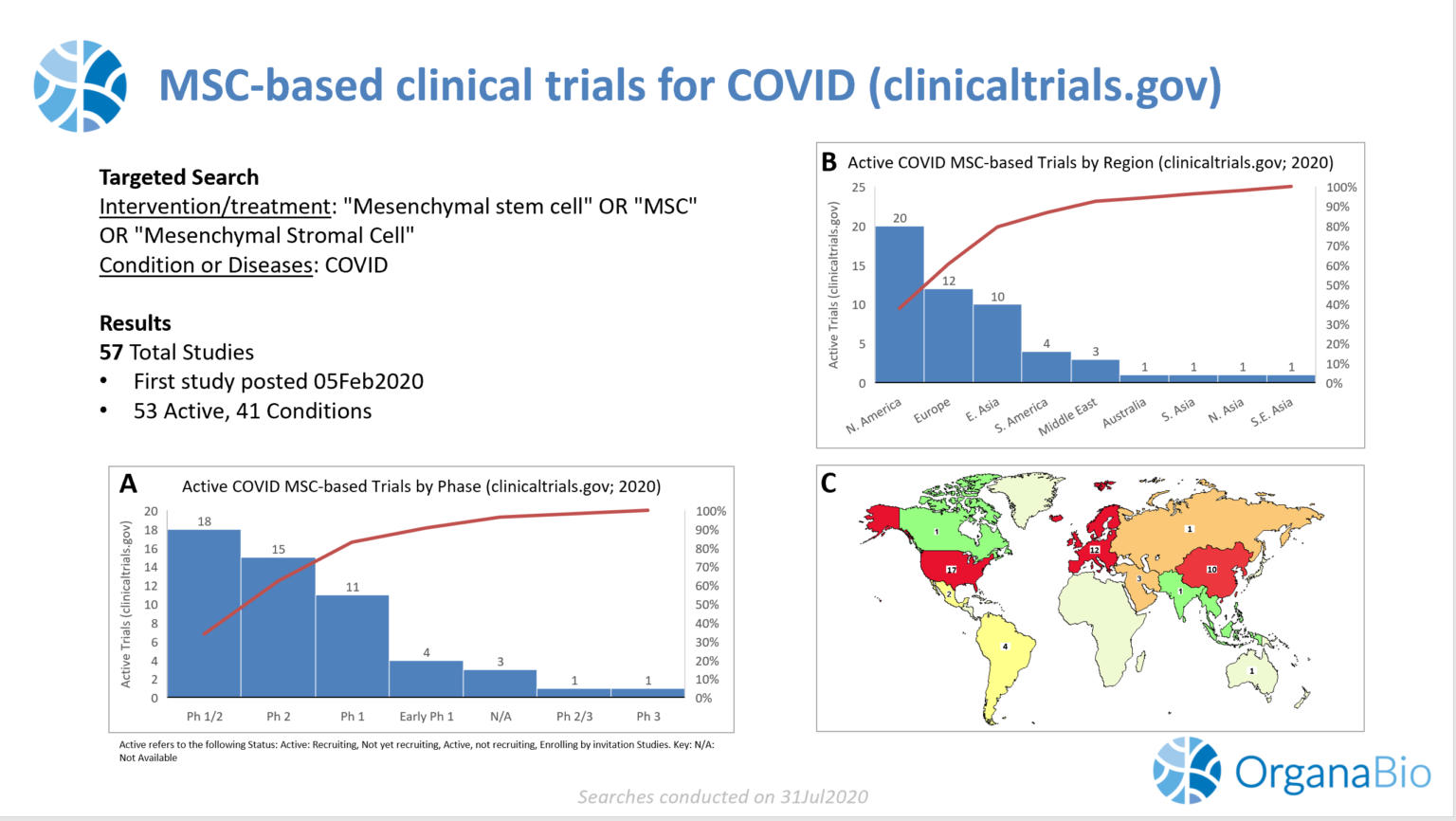
Figure 1 (A-C) Summary of “Active” MSC-based therapies for COVID-19 on clinicaltrials.gov. For A and B, “Active” trials represent the following Status: Available; Recruiting; Active, not recruiting; Enrolling by invitation Studies. Red line represents the cumulative percent total of trials. A: “Active” COVID MSC-based trials by Phase. N/A represents trials whose Phase information was not readily available. B: “Active” COVID MSC-based trials by Region. C: Heat map representing the regional trial information summarized in B. All searches conducted on 31Jul2020.
Of the current active trials, two are in late-stage development. “Mesenchymal Stem Cell Therapy for SARS-CoV-2-related Acute Respiratory Distress Syndrome” (NCT04366063; Royan Institute|Tehran University of Medical Sciences|Shahid Beheshti University of Medical Sciences) is a randomized, parallel design Phase 2/3 trial evaluating the therapeutic effects of conventional therapy in conjunction with (1) intravenous infusions of MSCs alone or (2) intravenous infusion of MSCs in combination with intravenous infusion of MSC-derived extracellular vesicles (EVs). The therapeutic MSC dose will consist of two infusions of 1×108 MSCs/patient. For EVs, patients will receive two infusions; however, EV concentrations were not readily available on the trial’s page. Initially posted on April 28, 2020, the trial is currently recruiting 60 participants for its three cohorts.
“MSCs in COVID-19 ARDS” (NCT04371393; Icahn School of Medicine at Mount Sinai|Mesoblast, Inc.) is a randomized, double blind, parallel design, placebo controlled Phase 3 trial evaluating the safety and efficacy of conventional ARDS therapy in conjunction with Mesoblast’s allogeneic bone marrow-derived MSC product, remestemcel-L. The therapeutic dose for this trial will consist of two infusions of 1×106 MSCs/kg body weight. Initially posted on May 1, 2020, the trial is currently recruiting 300 participants for its two cohorts.
Also of significance, is the only expanded access trial for COVID-19 in adolescents: “Intermediate-size Expanded Access Program (EAP), Mesenchymal Stromal Cells (MSC) for Multisystem Inflammatory Syndrome in Children (MIS-C) Associated With Coronavirus Disease (COVID-19)” (NCT04456439; Mesoblast, Ltd. [Mesoblast International Sàrl]). As defined by the clinicaltrials.gov website, expanded access trials (commonly also referred to as “compassionate use”) are “A way for patients with serious diseases or conditions who cannot participate in a clinical trial to gain access to a medical product that has not been approved by the U.S. Food and Drug Administration (FDA)”. Additional information regarding expanded access trials can be found on the FDA’s website, here. This trial is extremely significant because adolescents are now known to affected by the COVID-195, but due to their age, aren’t normally represented in clinical trials. The therapeutic dose for Mesoblast sponsored trial could consist of up to two infusions of 2×106 MSCs (remestemcel-L). Initially posted on July 2, 2020, the expanded access trial is currently available and is expected to treat around 50 participants between 2 months to 17 years of age.
If positive outcomes are seen in these late-stage trials, we can fully expect to see the rapid emergence of additional MSC-based clinical trials for COVID-19. While our searches focused on the clinicaltrials.gov database, other resources like Cell Trials Data (www.celltrials.org) can provide you with a broader view of global, cellular-based clinical trials for COVID-19. We encourage you visiting the celltrials.org site and we welcome any feedback on other databases we should check out. Given the promising data so far and the rapid advancement of these trials, we will continue to revisit the clinicaltrials.gov database monthly to chart progress and update these metrics. Check in next month for an updated version of this post!
References
- Golchin, A., Seyedjafari, E., & Ardeshirylajimi, A. (2020). Mesenchymal stem cell therapy for COVID-19: Present or future. Stem Cell Reviews and Reports, 16(3), 427-433.
- Leng, Z., Zhu, R., Hou, W., Feng, Y., Yang, Y., Han, Q., . . . Zhao, R. C. (2020). Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging and Disease, 11(2), 216-228.
- Bari, E., Ferrarotti, I., Saracino, L., Perteghella, S., Torre, M. L., & Corsico, A. G. (2020). Mesenchymal stromal cell secretome for severe COVID-19 infections: Premises for the therapeutic use. Cells, 9(4).
- World Health Organization. Novel coronavirus—China. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china
- Qiu, H., Wu, J., Hong, L., Luo, Y., Song, Q., & Chen, D. (2020). Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in zhejiang, china: An observational cohort study. The Lancet Infectious Diseases, 20(6), 689-696.






