Natural Killer (NK) cells have been considered a part of the innate immune system due to their lack of antigen specific receptors. Unlike other lymphocytes that develop in the bone marrow from lymphoid progenitor cells, NK cells are large with distinctive granules containing perforin and granzymes. The cytotoxic granules bind and penetrate target cells to induce cell death. NK cells also transmit death signals by cytokines (such as Fas Ligand and Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TRAIL) in order to kill infected and cancer cells which prompts subsequent immune reactions. Another unique killing ability that NK cells have is antibody-dependent cell-mediated cytotoxicity (ADCC), which is triggered by NK’s Fc receptor (CD16) interaction with an antibody bound to a cell. NK cells ability to detect and destroy tumor cells and virally-infected cells makes them an important component of the immune system.
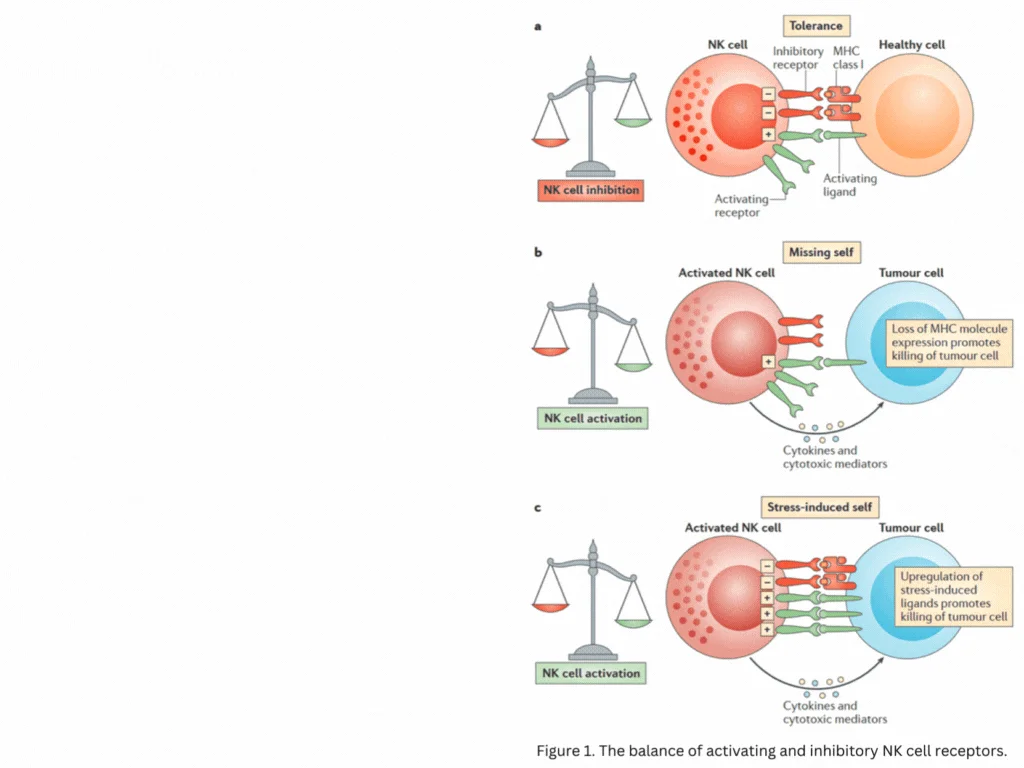
NK cells are activated in response to interferons or cytokines (such as IL-2 and IL-15) that are secreted by other immune cells early in many pathogenic infections. However, the mechanism by which NK cells distinguish between infected and uninfected cells is not completely understood. NK cells use several receptors that recognize self-molecules and non-self or ‘altered’ self-molecules. Killing is induced by ‘activating receptors’ (for example, NKG2D) and inhibited by ‘inhibitory receptors’, also called killer immunoglobulin-like receptors (KIRs). KIRs are specific for MHC class I molecules, which may explain why NK cells are able to kill targets with low levels of MHC class I. Reduced expression of MHC class I molecules is a mechanism used by many intracellular pathogens in order to evade destruction by T cells. Tumor cells can also exhibit decreased MHC class I molecules as an immune escape mechanism but makes them susceptible to NK cell killing (Figure 1). The unique ability of NK cells to kill with no prior antigen exposure and without MHC restriction requires a very tight regulation of activating and inhibitory receptors, but also makes these cells an attractive allogeneic cell therapy approach for immunotherapies.
One of the early anti-tumor effects of NK cells was realized after bone marrow transplant, as NK cells are among the first to reconstitute and demonstrate anti-malignant activity. In transplantation settings, NK cells also inhibit activation of graft-derived T cells, which helps limit the risk of graft versus host disease (GVHD). Since then, many studies utilized allogeneic NK cell infusions in non-transplant settings and achieved favorable outcomes in cancer patients. In certain studies, NK cells are activated ex-vivo and are supported in-vivo by IL-2 or IL-15 injections. These studies concluded that treatment with NK cells is safe, well tolerated, effective. Genetically-modified NK cells are also being utilized as immunotherapies. The NK cell line, NK-92 has been engineered to express CARs, such as Nkarta’s NKG2D CAR-NK, and NantKwest’s high-affinity Fc receptor/CD16 (haNK) to promote ADCC. Additionally, anti-CD19 CAR-NK cells are being investigated in treatment against CD19-expressing hematological malignancies by Takada.
It is yet to be determined what are the critical factors that make or break an NK cell product for immunotherapy. That is why, OrganaBio provides high-quality, highly characterized (immunophenotyping, HLA and KIR), NK cells, isolated freshly from various tissue sources. We focus on manufacturing NK cell products with a clear path to the clinic and continue to establish and grow a robust donor database.
References
- Calabrese DR, Lanier LL, Greenland JR. Natural killer cells in lung transplantation. Thorax. 2019 Apr;74(4):397-404. doi: 10.1136/thoraxjnl-2018-212345.
- Vivier, E., Ugolini, S., Blaise, D. et al. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 12, 239–252 (2012).
- Shah N, Li L, McCarty J, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol. 2017;177(3):457-466. doi:10.1111/bjh.14570
- Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J. The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments. Front Immunol. 2017 May 31;8:631. doi: 10.3389/fimmu.2017.00631. PMID: 28620386; PMCID: PMC5450018.
- Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. 2017 Jun;31:37-54. doi: 10.1016/j.smim.2017.07.009. Epub 2017 Aug 31. PMID: 28838796.
- Wagner AK, Alici E, Lowdell MW. Characterization of human natural killer cells for therapeutic use. Cytotherapy. 2019 Mar;21(3):315-326. doi: 10.1016/j.jcyt.2018.11.001. Epub 2019 Mar 23. PMID: 30910383.






