OrganaBio’s trial support services are focused on fast and efficient processing of blood samples from clinical trial patients to isolate peripheral blood mononuclear cells (PMBCs) before cryopreservation, cold storage, and shipping to the Sponsor’s lab. With bicoastal CPC Services located in Irvine and Miami and planned expansions into Chicago, Ann Arbor, Nashville, and Atlanta, we aim to establish local processing sites to support clinical research locations lacking PBMC processing capabilities and to shorten the time from sample collection to processing and cryopreservation. We also provide access to highly diverse populations of clinical trial participants and donor tissues.

With a bicoastal presence and planned expansions in the Midwest and Southeast, OrganaBio offers quick turnaround times, flexibility, and customization – including a variety of solutions to meet your program’s unique needs.








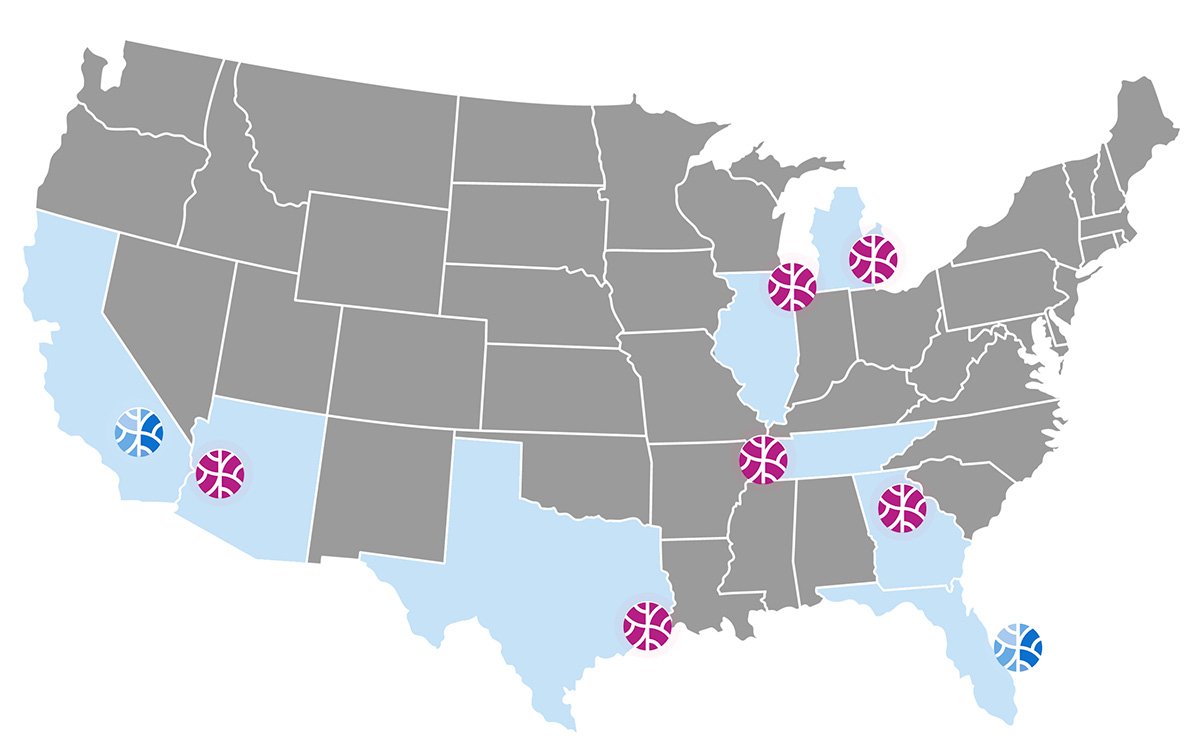
OrganaBio’s unique Hub Processing model is the cornerstone of Sponsors’ clinical trial success. It ensures fast and efficient sample processing and cryopreservation. Our exceptional track record, an experienced, trained, and qualified team, and a consistent quality management system across geographies ensure efficient technology transfer and proven accuracy. OrganaBio’s client-centric and data-driven approach enables seamless operations between Sponsors, clinical sites, and OrganaBio’s dedicated staff.

©2025 OrganaBio. All rights reserved.