Simple Science: Cardiac Regenerative Medicine
The field of cardiac regenerative medicine is rapidly developing solutions to treat the millions of people around the world affected by cardiac illnesses. The articles summarized in the Simple Science posts below outline a few of the recent advancements in cardiac tissue engineering, disease modeling, and the role of the stem cell secretome in cardiac protection. OrganaBio’s goal in summarizing these complex articles is to translate regenerative medicine articles that excite our scientists into articles that anyone can enjoy, whether they’re looking for information that inspires hope or just looking for a good read. Scientific articles may be written for a research-savvy audience but, in the end, the discoveries they outline affect us all. We hope you enjoy some Simple Science!
Simple Science 1: 3D Printing Heart Tissue Using Induced Pluripotent Stem Cells
Article Title: In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid
Authors: Molly E. Kupfer, Wei-Han Lin, Vasanth Ravikumar, Kaiyan Qiu, Lu Wang, Ling Gao, Didarul B. Bhuiyan, Megan Lenz, Jeffrey Ai, Ryan R. Mahutga, DeWayne Townsend, jianyi Zhang, Michael C. McAlpine, Elena G. Tolkacheva, Brenda M. Ogle
Link to Full Article: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.119.316155
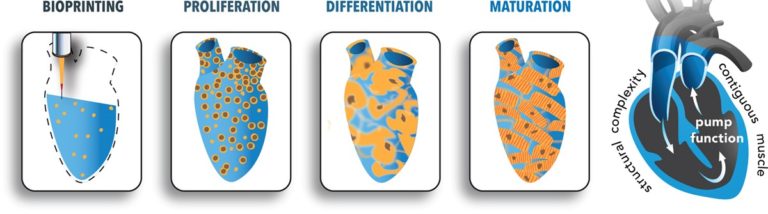
These researchers 3D-printed heart tissue. 3D printing of human cells is an emerging and exciting field. Special 3D printers are loaded with cells that have been mixed with liquid “bio-ink” that, after printing, turns into a solid.
In this article, researchers aimed to print cardiac muscle tissue. Their approach started with induced pluripotent stem cells, which are stem cells created from almost any cell in the body. In this case, the stem cells were created from human fibroblasts. The plan from there was to reprogram the stem cells into heart cells and print them, along with the bio-ink, into a structure upon which they can grow.
The problem was that they just couldn’t seem to get enough cells to grow and organize together. So, they flipped their approach. Instead of reprogramming the stem cells into heart cells before printing, they printed the cells into the structure and then reprogrammed them into heart cells. Before they knew it, the heart cells they had printed were actually beating! The cells were reprogrammed next to one another on the scaffold, so it was easier for them to organize and work together.
These findings can be used to:
- Print tissue to be used as an implant on damaged hearts
- Make a heart model to test treatments
Congrats to these researchers on their findings!
Simple Science 2: Clinical Trial in a Dish using iPSCs
Article Title: Clinical trial in a dish using iPSCs shows lovastatin improves endothelial dysfunction and cellular cross-talk in LMNA cardiomyopathy
Authors: Nazish Sayed, Chun Liu, Mohamed Ameen, Farhan Himmati, Joe Z. Zhang, Saereh Khanamiri, Jan-Renier Moonen, Alexa Wnorowski, Linling Cheng, June-Wha Rhee, Sadhana Gaddam, Kevin C. Wang, Karim Sallam, Jack H. Boyd, Y. Joseph Woo, Marlene Rabinovitch, Joseph C. Wu
Link to Full Article: https://pubmed.ncbi.nlm.nih.gov/32727917/
These Stanford researchers are very clever. They set out to find a treatment for dilated cardiomyopathy (DCM), a disease that stunts the heart’s ability to pump blood. The best way to test a treatment for this disease would be to acquire some heart tissue and apply the therapeutic to it. Unfortunately, you’d be hard-pressed to find donors willing to have open-heart surgery so researchers could extract some tissue to test treatments that only have a chance of working.
So these researchers thought of another way. They took cells from a group of patients with DCM, and a group of healthy patients. They converted those cells into induced pluripotent stem cells (iPSCs), stem cells that can be made from almost any cell in the body.
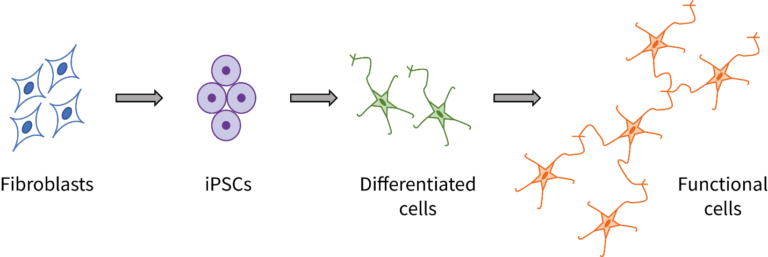
They took those iPSCs and converted them into endothelial cells, which are cells involved in the mechanism that causes dilated cardiomyopathy. So, cells from patients –> iPSCs –> endothelial cells (one group from healthy patients, one from DCM patients). Now the researchers have cells upon which they can test their treatment, no tissue-harvesting necessary. They performed testing and found a drug that works to restore normal function in the endothelial cells.
Millions of dollars and months of time saved – all thanks to stem cells!
Simple Science 3: Mesenchymal Stromal Cell-Secreted Signals for Cardiac Protection
Article Title: Human pluripotent stem cell-derived cardiomyocytes as a target platform for paracrine protection by cardiac mesenchymal stromal cells
Authors: Chrystalla Constantinou, Antonio M. A. Miranda, Patricia Chaves, Mohamed Bellahcene, Andrea Massaia, Kevin Cheng, Sara Samari, Stephen M. Rothery, Anita M. Chandler, Richard P. Schwarz, Sian E. Harding, Prakash Punjabi, Michael D. Schneider, Michela Noseda
Link to Full Article: https://www.nature.com/articles/s41598-020-69495-w#Sec10
This is the first time that the secretome of human cardiac mesenchymal stromal cells (MSCs) has been shown to suppress human cardiac cell death.
In this Simple Science, worlds collide!
3 important cell therapy components were used in this experiment.
- iPSCs – stem cells made from other bodily cells
- MSCs – stem cells present in the body
- Exosomes – signaling packages secreted by cells
It’s been shown that other cells in the body are protected from cell death when MSCs are injected into the body. Some believe it’s the secretome (all the things secreted by MSCs) that’s doing the protecting. Others believe it’s just the exosomes from the secretome.
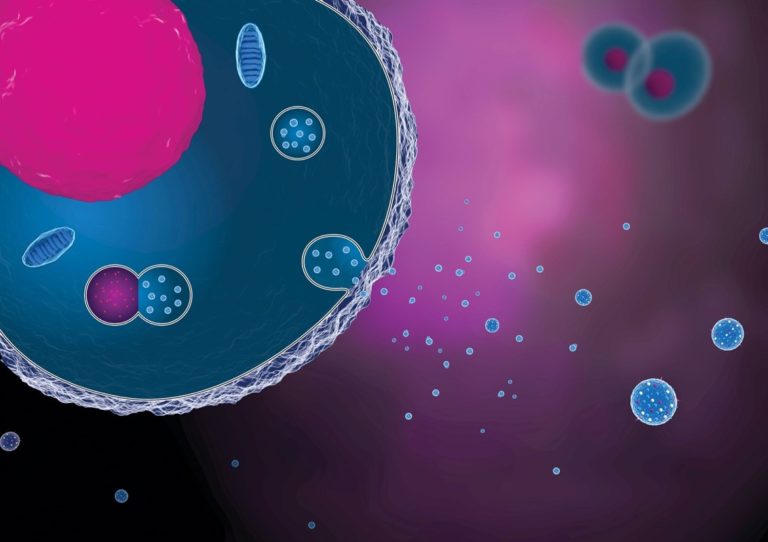
Figure 3 – Conceptual Rendering of Exosomes/Extracellular Vesicles (Source: GEN)
In order to test this out, the researchers took iPSCs and converted them into cardiac muscle cells to use as a testing model of the heart. Cardiac MSCs were grown in a dish with medium (growth liquid), capturing the secretome in the medium. The researchers took the medium from the dish and combined it with the heart cell model for their first test group. They combined only exosomes from the secretome with the heart cell model for the second test group. The researchers simulated cardiac injury and, in the end, found that the exosomes alone didn’t do the job. The entire secretome of the MSCs was required to protect the heart cells from death.
Incredible findings by these researchers!
Takeaways
Cardiac regenerative medicine is a rapidly developing field worth paying attention to. Understanding the creativity and innovative attitude with which researchers approach their work inspires hope for those in need of treatments. OrganaBio is glad to be able to provide these summaries both for people who are reading them for leisure, and for those reading them to find solace in knowing that some of the world’s smartest minds are working to keep them healthy.
References:
SS1: Kupfer, M. E., Lin, W., Ravikumar, V., Qiu, K., Wang, L., Gao, L., . . . Ogle, B. M. (2020). In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circulation Research, 127(2), 207-224. doi:10.1161/circresaha.119.316155
SS2: Sayed N, Liu C, Ameen M, et al. Clinical trial in a dish using iPSCs shows lovastatin improves endothelial dysfunction and cellular cross-talk in LMNA cardiomyopathy. Sci Transl Med. 2020;12(554):eaax9276. doi:10.1126/scitranslmed.aax9276
SS3: Constantinou, C., Miranda, A.M.A., Chaves, P. et al. Human pluripotent stem cell-derived cardiomyocytes as a target platform for paracrine protection by cardiac mesenchymal stromal cells. Sci Rep 10, 13016 (2020). https://doi.org/10.1038/s41598-020-69495-w
Image Sources:
Figure 1: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.119.316155






