Cord blood is a rich source of hematopoietic stem cells (HSCs), which are immature cells that can develop into all types of blood cells. This makes HSC-rich umbilical cord blood an ideal starting material for transplantation and cell therapy. Cord blood HSCs are already approved and have been used in Hematopoietic Stem Cell Transplantation (HSCT) since 1988 for treating blood cancers, like leukemia and lymphoma, and other blood and immune system disorders, like aplastic anemia and sickle cell disease.1,2 More recently, cord blood HSCs are being further explored in research and clinical studies for a variety of purposes; for example, studies suggest cord blood HSCs might improve motor function and brain connectivity in cerebral palsy or stroke, and early research shows promise for cord blood HSCs to potentially address the underlying cause of hearing loss. Compared to bone marrow and peripheral blood stem cells, and due to their immaturity, cord blood HSCs are less likely to trigger Graft-versus-Host Disease (GVHD) and have less stringent HLA matching requirements, increasing the pool of compatible donors; therefore, cord blood HSCs can be used to treat a wider range of patients than stem cells from other sources, because they are less likely to be rejected by the recipient’s immune system.3,4 Additionally, cord blood HSCs can engraft, or take hold, in the recipient’s body more quickly than HSCs derived from bone marrow.3,4
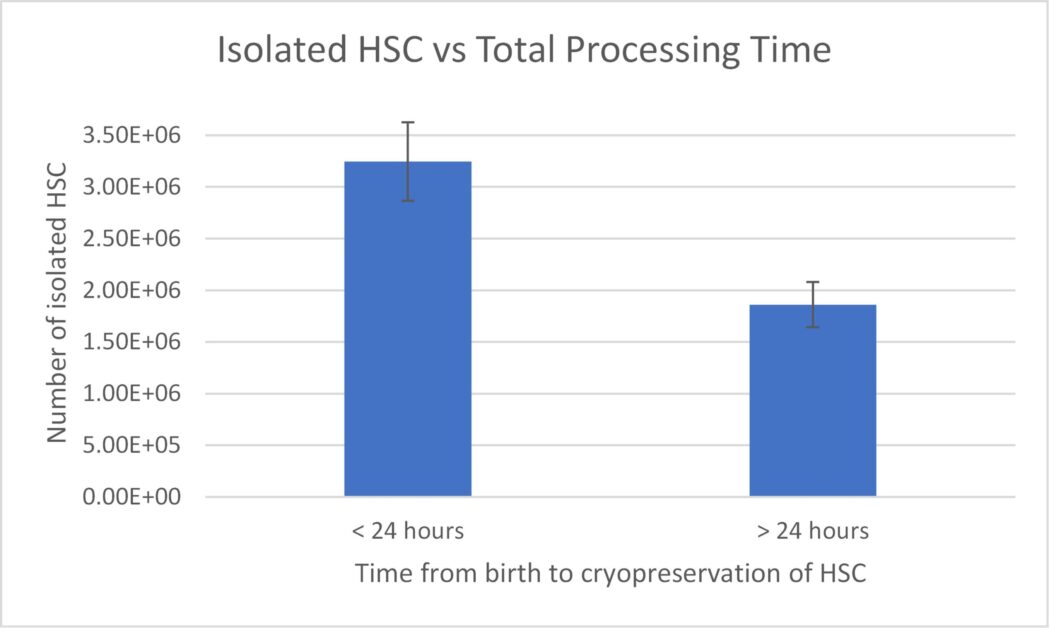
Cord blood is collected immediately after a birth with minimal risk to the mother or baby, and HSCs are isolated from the blood using established selection methods.5,6 Cord blood HSCs can be stored for long periods of time in vapor phase liquid nitrogen (LN2), which, in addition to their biological characteristics, makes them a readily available and attractive source of stem cells for transplantation or cell therapies. However, a limiting factor of cord blood is the low volume of blood, and therefore, the low number of HSCs in a single unit, which makes this cell population scarce and difficult to isolate. The success of HSC isolation from umbilical cord blood depends heavily on two factors: quality and quantity, and unfortunately, both can vary significantly from donor to donor. The source variability depends on factors like the volume of cord blood collected and the total number of nucleated cells (TNC) present. In addition to these factors, our data (Figure 1) and data recently published by Katy Rezvani’s group at MD Anderson suggest that successful isolation and high yield of HSCs significantly depend on time from tissue collection to HSC isolation and cryopreservation.7 Fresh cord blood processing must begin as soon after delivery as possible, but no later than 20-24 hours post-birth. This ties in nicely to recent findings published in Nature Medicine, which show CAR-NK cells from cord blood units with collection to cryopreservation time of ≤ 24 hours correlate with superior efficacy and optimal therapeutic outcome.7
OrganaBio’s cord blood-derived HSCs are isolated from fresh cord blood units, sourced through its wholly owned, FDA-registered subsidiary, GaiaGift. The OrganaBio team works closely with consented donors and trained physicians to ensure quality and consistency at every step of the process, from donor recruitment and tissue collection to product manufacturing and distribution. A key advantage for OrganaBio’s business model is the co-location of practices through which cord blood units are sourced and its GMP manufacturing facility in South Florida. This proximity not only allows for full chain of custody control and oversight, but also for immediate processing, leading to higher yields of viable HSCs.
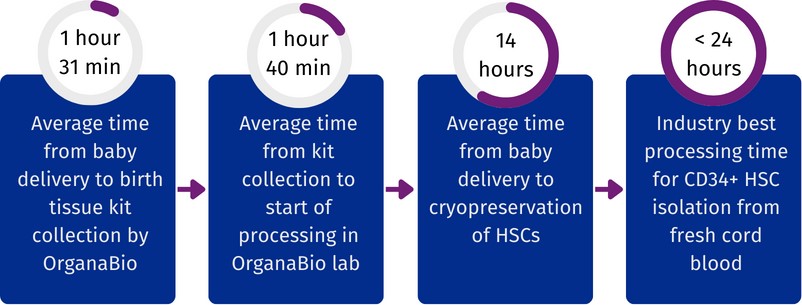
As illustrated in Figure 2, to date, the average time from delivery to birth tissue kit collection is 1 hour 31 minutes, the average time from birth tissue kit collection to the start of HSC processing is 1 hour 40 minutes, and the average time from birth to cryopreservation of isolated HSCs is <14 hours. Thus, OrganaBio HSCs are isolated from cord blood units for which total processing time (time from birth to cryopreservation of HSCs) is much less than the critical 24-hour window.
The use of cord blood for cell and gene therapies offers a beacon of hope for treating a wide range of diseases. Cord blood-derived cell therapies are an attractive alternative for adult peripheral blood due to easier availability, wider matching range, and faster engraftment. However, for this potential to be realized, access to high-quality cord blood units and subsequent robust, reproducible high yields of clinically-relevant or GMP compliant HSCs are crucial to therapeutic program success. By working to overcome challenges related to cord blood quality, quantity, and processing timelines, we can unlock the full potential of HSCs derived from umbilical cord blood.
This is where OrganaBio’s commitment to data-driven decision making and excellence comes in. OrganaBio’s extensive tissue sourcing, collection, and manufacturing expertise are backed by over five years of data collection and analyses. All cord blood units undergo rigorous evaluation to ensure they meet the strictest standards for optimized manufacturing. This, along with adhering to highest ethical standards, will significantly contribute to advancing stem cell-based therapies and offer new hope to millions battling blood cancers and a variety of other diseases and disorders.
References
-
- Broxmeyer HE, Gluckman E, Auerbach A, Douglas GW, Friedman H, Cooper S, Hangoc G, Kurtzberg J, Bard J, Boyse EA. Human umbilical cord blood: a clinically useful source of transplantable hematopoietic stem/progenitor cells. Int J Cell Cloning. 1990 Jan;8 Suppl 1:76-89; discussion 89-91. doi: 10.1002/stem.5530080708. PMID: 1969886.
- Ropa J, Van’t Hof W. The fulfilled promise and unmet potential of umbilical cord blood. Curr Opin Hematol. 2024 Jul 1;31(4):168-174. doi: 10.1097/MOH.0000000000000817. Epub 2024 Apr 4. PMID: 38602152.
- Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, McGlave PB, Wagner JE. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007 Oct 15;110(8):3064-70. doi: 10.1182/blood-2007-04-067215. Epub 2007 Jun 14. PMID: 17569820; PMCID: PMC2018678.
- van Besien K, Hari P, Zhang MJ, Liu HT, Stock W, Godley L, Odenike O, Larson R, Bishop M, Wickrema A, Gergis U, Mayer S, Shore T, Tsai S, Rhodes J, Cushing MM, Korman S, Artz A. Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and graft-versus-host disease-free, relapse-free survival. Haematologica. 2016 May;101(5):634-43. doi: 10.3324/haematol.2015.138594. Epub 2016 Feb 11. PMID: 26869630; PMCID: PMC5004373.
- Farley TJ, Ahmed T, Fitzgerald M, Preti RA. Optimization of CD34+ cell selection using immunomagnetic beads: implications for use in cryopreserved peripheral blood stem cell collections. J Hematother. 1997 Feb;6(1):53-60. doi: 10.1089/scd.1.1997.6.53. PMID: 9112218.
- Denning-Kendall PA, Horsley H, Donaldson C, Bradley B, Hows JM. Different behaviour of fresh and cultured CD34+ cells during immunomagnetic separation. Br J Haematol. 1999 Jun;105(3):780-5. doi: 10.1046/j.1365-2141.1999.01397.x. PMID: 10354147.
- Marin D, Li Y, Basar R, Rafei H, Daher M, Dou J, Mohanty V, Dede M, Nieto Y, Uprety N, Acharya S, Liu E, Wilson J, Banerjee P, Macapinlac HA, Ganesh C, Thall PF, Bassett R, Ammari M, Rao S, Cao K, Shanley M, Kaplan M, Hosing C, Kebriaei P, Nastoupil LJ, Flowers CR, Moseley SM, Lin P, Ang S, Popat UR, Qazilbash MH, Champlin RE, Chen K, Shpall EJ, Rezvani K. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat Med. 2024 Mar;30(3):772-784. doi: 10.1038/s41591-023-02785-8. Epub 2024 Jan 18. PMID: 38238616; PMCID: PMC10957466.






